- Home
- Product Center
- LC-100 HPLC
- LC-80 ChroMini HPLC
- EX1800 UHPLC/HPLC
- EX2000 MS LC-MS/MS
- ARCUS 5/7 AutoSampler
- Pump/ High Precision Pump
- Detector Units
- Purification System
- Gel Permeation Chromatography
- Post-column derivatization
- Workstation
- Consumable parts
- News
- Applications
- Customer Service
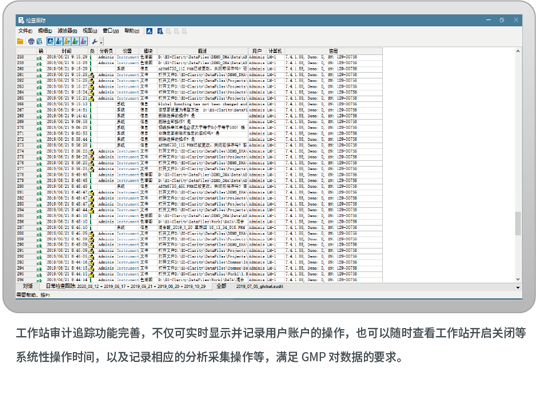
Workstation EX-CLARITYThe workstation conforms to data GLP certification,GMP certification, FDA certification
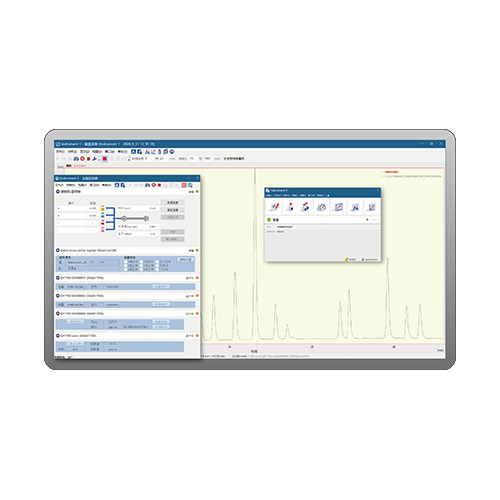
The software can collect one to four independent detector signals simultaneously. A variety of high precision 24-bit chromatography signal acquisition modes enables digital control of the instruments .You can establish a high performance general chromatographic workstation with multi-channel analysis and multi-user access.
The workstation conforms to data GMP certification, FDA certification, Installation Qualification/Operational Qualification (IQ/OQ), System Suitability Test (SST) and other specifications intended to ensure data validity and security. Efficient batch processing streamlines the whole process of instrument control, auto-sampler sequence acquisition, automatic integration calibration, and reporting output. It also features post-processing, chromatogram comparison, re-calibration, data input and output, and three-dimensional chromatogram processing.